The Weill Cornell Medicine/NewYork-Presbyterian Joint Clinical Trials Office (JCTO) is a centralized research administration resource providing comprehensive support for clinical trials to principal investigators and their teams in the following service areas:
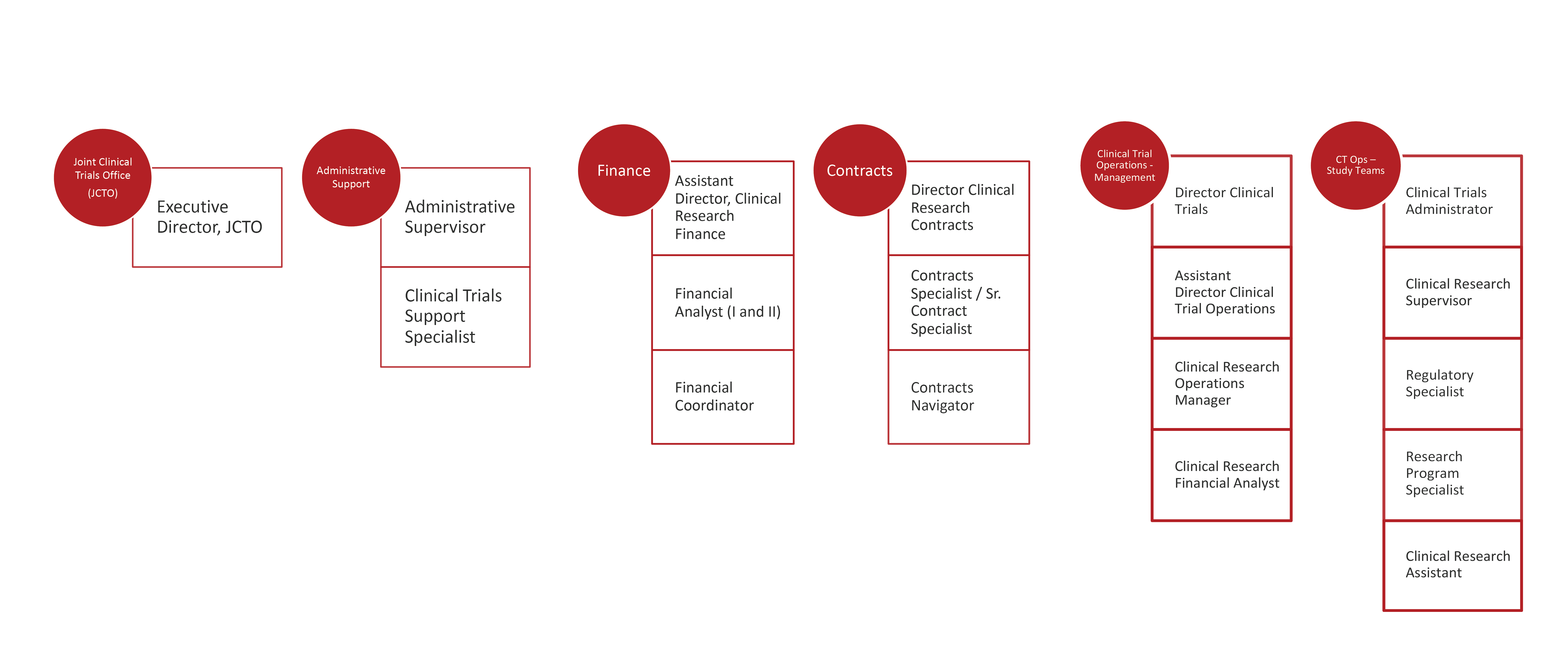
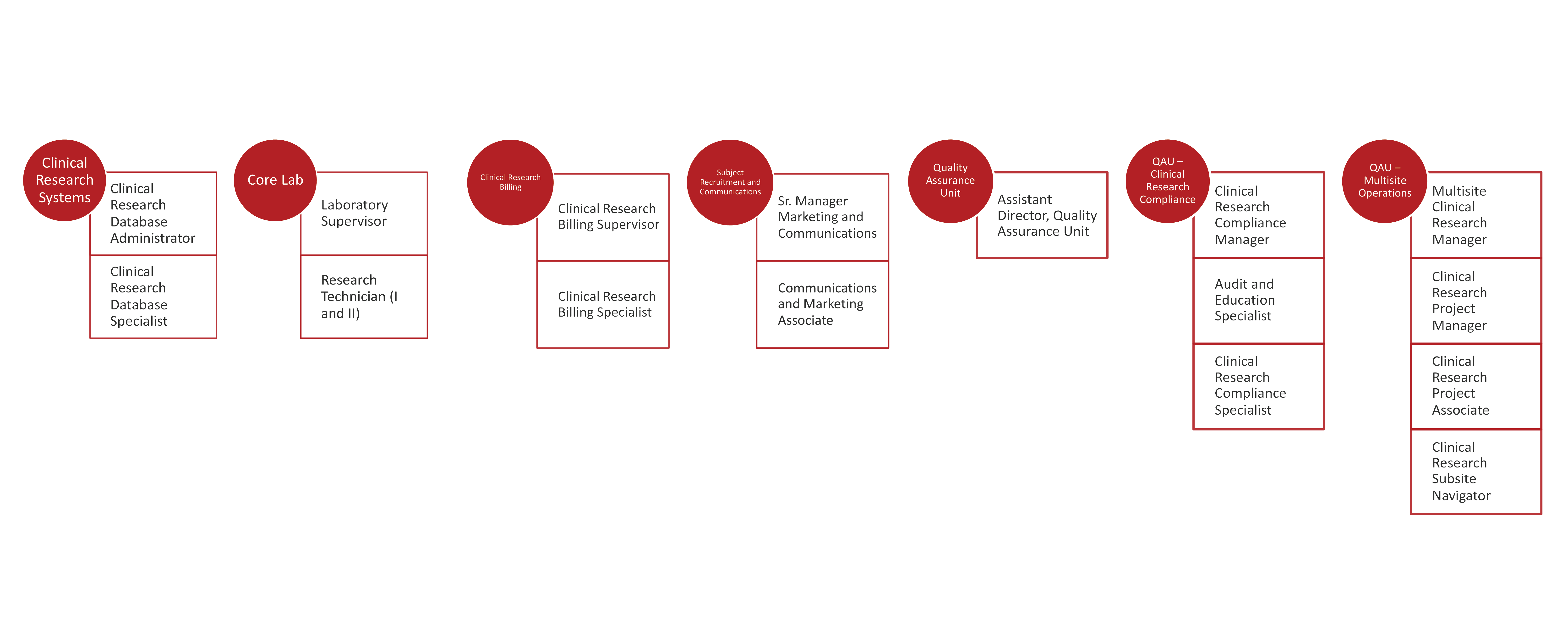
Sample Clinical Research positions include:
- Clinical Trials Administrator
- Regulatory Specialist
- Regulatory and Data Specialist
- Regulatory Coordinator
- Data Coordinator
- Data Control Assistant
- Research Coordinator
- Research Aide
- Assistant Research Coordinator
Open positions:
- Research Program Specialist (Requestion ID: 86879)
- Clinical Research Operations Manager (Requisition ID: 86961)
- Clinical Research Compliance Specialist (Requestion ID: 85409)
- Research Program Specialist – QAU (Requestion ID: 86804)
- Research Program Specialist – QAU (Requisition ID: 86803)
To apply for positions with the JCTO, access Weill Cornell Medicine’s job search page > left side navigation > organization > division > Joint Clinical Trials Office. Or you can search on keywords in the free text field.
All applications must be submitted via the Weill Cornell Medicine careers page.



