Pfizer COVID-19 vaccine appointments are available to our patients. Sign up for Connect today to schedule your vaccination.
NEW YORK (September 11, 2015) — The Minimally Invasive New Technologies Program (MINT) at Weill Cornell Medical College and NewYork- Presbyterian Hospital has teamed up with entrepreneurs and investors to establish Lumendi, a new start-up company dedicated to producing next-generation endoscopic tools that make complex gastrointestinal surgeries safer and less expensive while also improving patient outcomes.
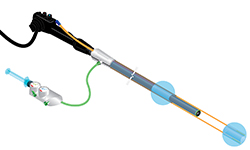
The agreement between Weill Cornell and Lumendi will advance the development of the Endolumenal Surgical Platform (ESP), a new disposable device which fits over a standard endoscope like a sleeve and provides increased stability, control and visualization within the intestine. These enhanced features are designed to allow clinicians to remove large polyps and eventually to treat strictures, fistulas and many types of inflammatory bowel disease without necessitating open or laparoscopic surgery. The ESP is designed to transform the way gastrointestinal surgeries are performed by enabling clinicians to perform complex procedures endolumenally — from entirely within the intestine — resulting in less-invasive surgeries, quicker patient recovery and reduced healthcare costs.
Lumendi will seek to transform the ESP prototype developed at MINT into a commercial product and seek clearance by the U.S. Food and Drug Administration. In collaboration with Lumendi, the MINT team plans to develop a series of new products that are designed to enhance the ESP.
"We are defining a new era of digestive surgery, and we believe that the ESP will pave the way," said Dr. Jeffrey Milsom, chief of Colon and Rectal Surgery at Weill Cornell Medical College and NewYork-Presbyterian/Weill Cornell Medical Center and MINT co-director, who holds an equity interest in Lumendi, Ltd. and serves as a paid advisor to the company. "The ESP device and ancillary tools are all relatively simple, and clinicians who use them should be quickly able to take a leap forward in terms of what they're able to do inside the intestine."
The ESP design features two balloons which create a therapeutic zone inside the intestine, allowing clinicians to stretch out the intestinal wall and straighten folds and bends in order to stabilize and better visualize the colon. The design also permits stabilizing the endoscope tip at the site of the disease, allowing clinicians to target their movements precisely.
"Current endoscopes don't offer the same level of stability that ESP does," said Dr. Milsom, who is also executive director of the Center for Advanced Digestive Care at Weill Cornell and NewYork-Presbyterian/Weill Cornell Medical Center, and the Jerome J. DeCosse M.D. Distinguished Professor of Surgery at Weill Cornell Medical College. "With this device, you can better control and manipulate the surgical environment inside the intestine."
As part of the agreement, MINT plans to develop a series of new products to enhance the ESP platform and increase the number of procedures that can be performed endolumenally. These tools include flexible surgical instruments that will enable precise complex surgical procedures to be conducted within the intestinal channel.
"Creating Lumendi has opened up many new possibilities for advancing gastrointestinal surgeries," said Dr. Peter Johann, chairman and CEO of Lumendi. "This innovative relationship has the potential to transform digestive care for patients around the world."
Lumendi, Ltd. holds an exclusive worldwide license from Cornell University on the ESP platform and related ancillary products. According to Dr. Johann, Lumendi's vision is to revolutionize digestive surgery by developing and making available tools and devices enabling minimally invasive gastrointestinal interventions. Formed in December 2014, Lumendi is collaborating with MINT for the further development of these devices. For more information, visit www.lumendi.com and www.mint.weill.cornell.edu.
Posted September 11, 2015 9:54 AM | Permalink to this post